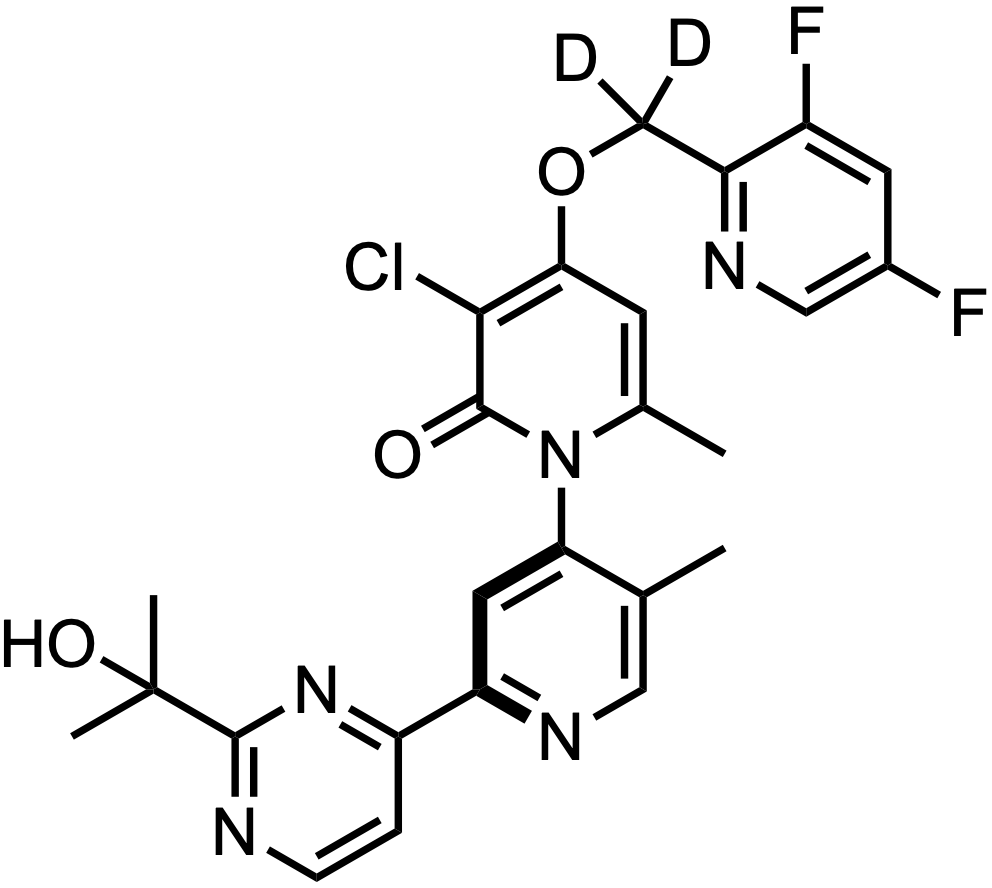
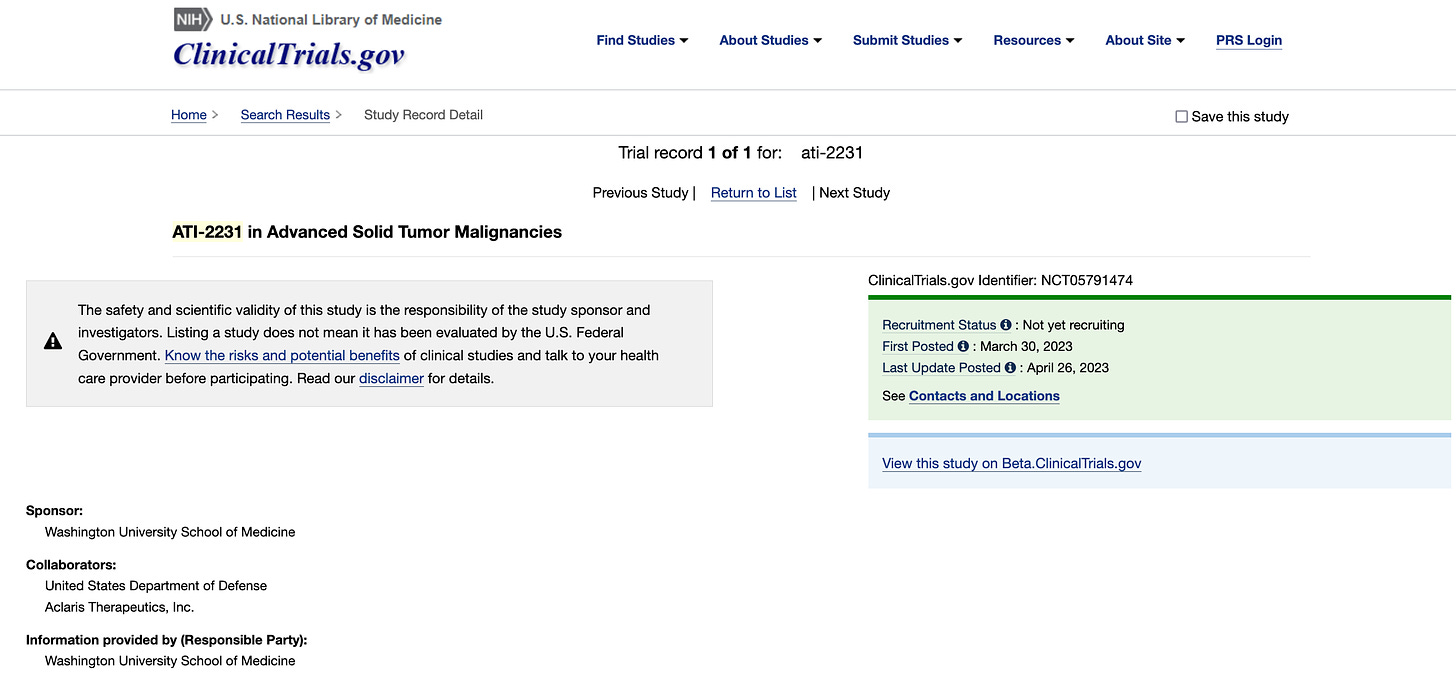
ATI-2231 is an oral MAP kinase-activated protein kinase 2 (MK2) inhibitor being developed by Aclaris Therapeutics (ACRS 0.00%↑) for the treatment of metastatic breast cancer and pancreatic cancer. While their pipeline site says it’s still preclinical, the company is co-sponsoring a phase 1 investigator initiated trial at WUSTL in advanced solid tumors (NCT05791474).
Why people think this is a good target
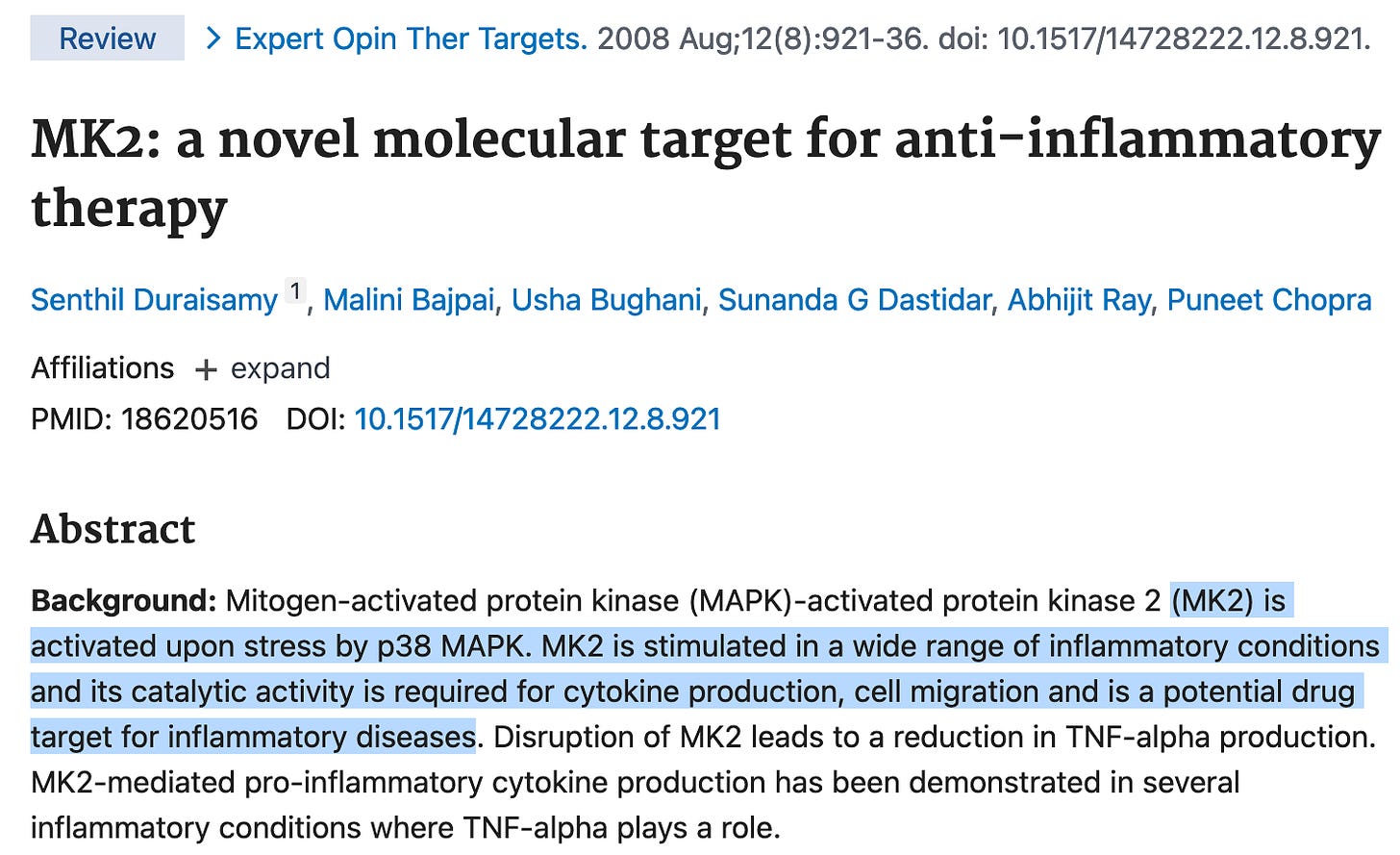
MK2 is thought to be a major node in the p38 mitogen-activated protein kinase (p38MAPK) pathway and MK2 inhibitors have been developed with a variety of inflammatory conditions in mind (rheumatoid arthritis, psoriatic arthritis, hidradenitis suppurativa, etc.)
So what does this have to do with Aclaris developing ATI-2231 for cancer?
In addition to inflammation, the p38MAPK pathway has been implicated in a variety of pathological conditions like metastasis. Explains the proposed indication for ATI-2231.
Getting acquainted (AKA, what are we dealing with here)
This compound was brought to my attention thanks to a subscriber! Yes, I do listen to your suggestions and try my best to fulfill requests where I can.
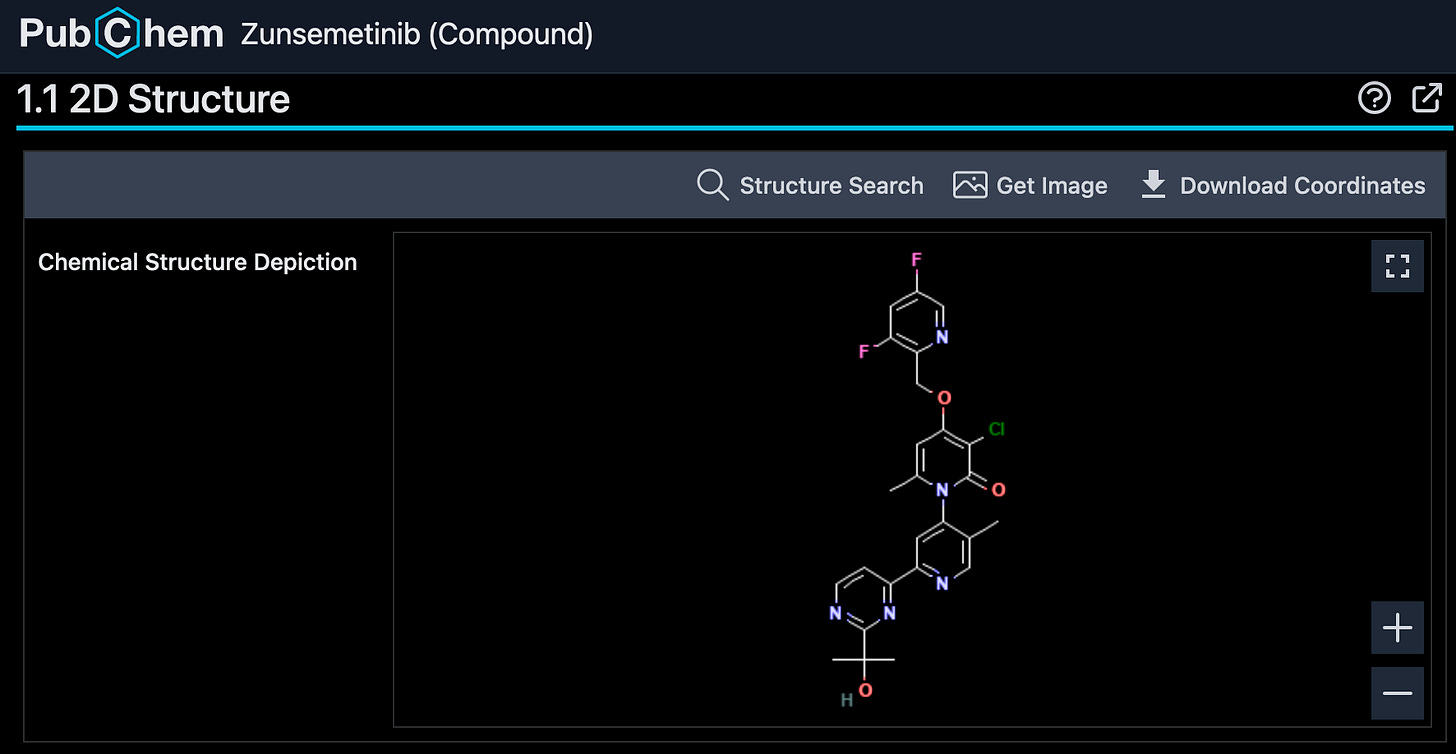
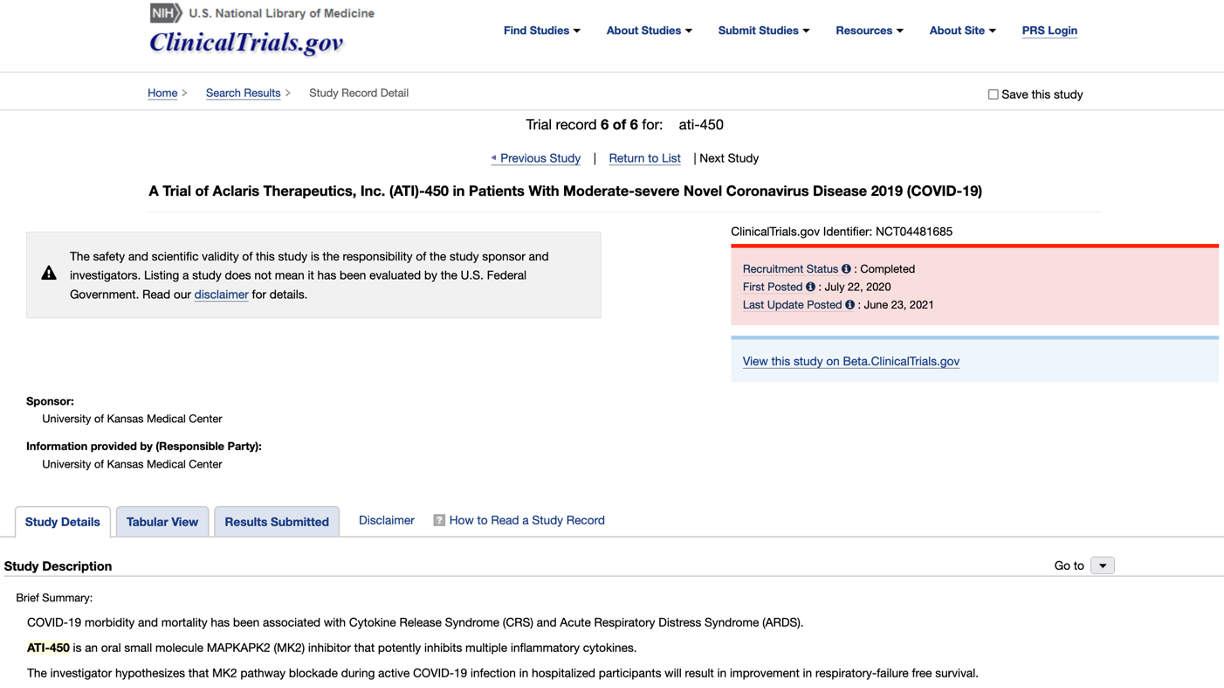
In acquainting myself with this space, I saw that Aclaris currently has an oral MK2 inhibitor, zunsemetinib (ATI-450), which is currently in phase 2 for rheumatoid arthritis and psoriatic arthritis (NCT05279417, NCT05511519).
ATI-450 was originally being trialed in phase 2 for the treatment of hidradenitis suppurativa, a painful skin condition (NCT05216224). But in March 2023, Aclaris announced that ATI-450 failed to meet its primary endpoint of improving inflammatory nodule/abscess count compared to placebo. But ATI-450 is still being trialed for severe rheumatoid arthritis (NCT05279417).
Anyway, I bring this up because this was my reference point when I went to search the patent space.
So to Espacenet I went! Searched “aclaris therapeutics”. Sorted by descending priority date and scanned the results for “MK2”.
I first encountered 3 patents—all of which seemed to describe ATI-450. One was on synthesis (WO2022109481A1), one was a process chemistry/forms patent (WO2021195475A1), and one was a methods of use for COVID treatment (bc everyone was throwing their compound at the virus back in the day lol… WO2021195507A1).

For the COVID patent, here’s some further proof that it’s talking about ATI-450 (if you don’t wanna do some clicking).
Yup, they really did that.
ANYWAY! My point is that these patents were zzZZ to me because I’m not looking for ATI-450—we already know what that looks like! I’m interested in finding clues for ATI-2231.
So I cast these patents aside. Bye.
I combed through the Espacenet hit list I’d gathered earlier in hopes of reeling something shiny in.
And shiny things I did find…
Deuteriums and atropoisomers galore!
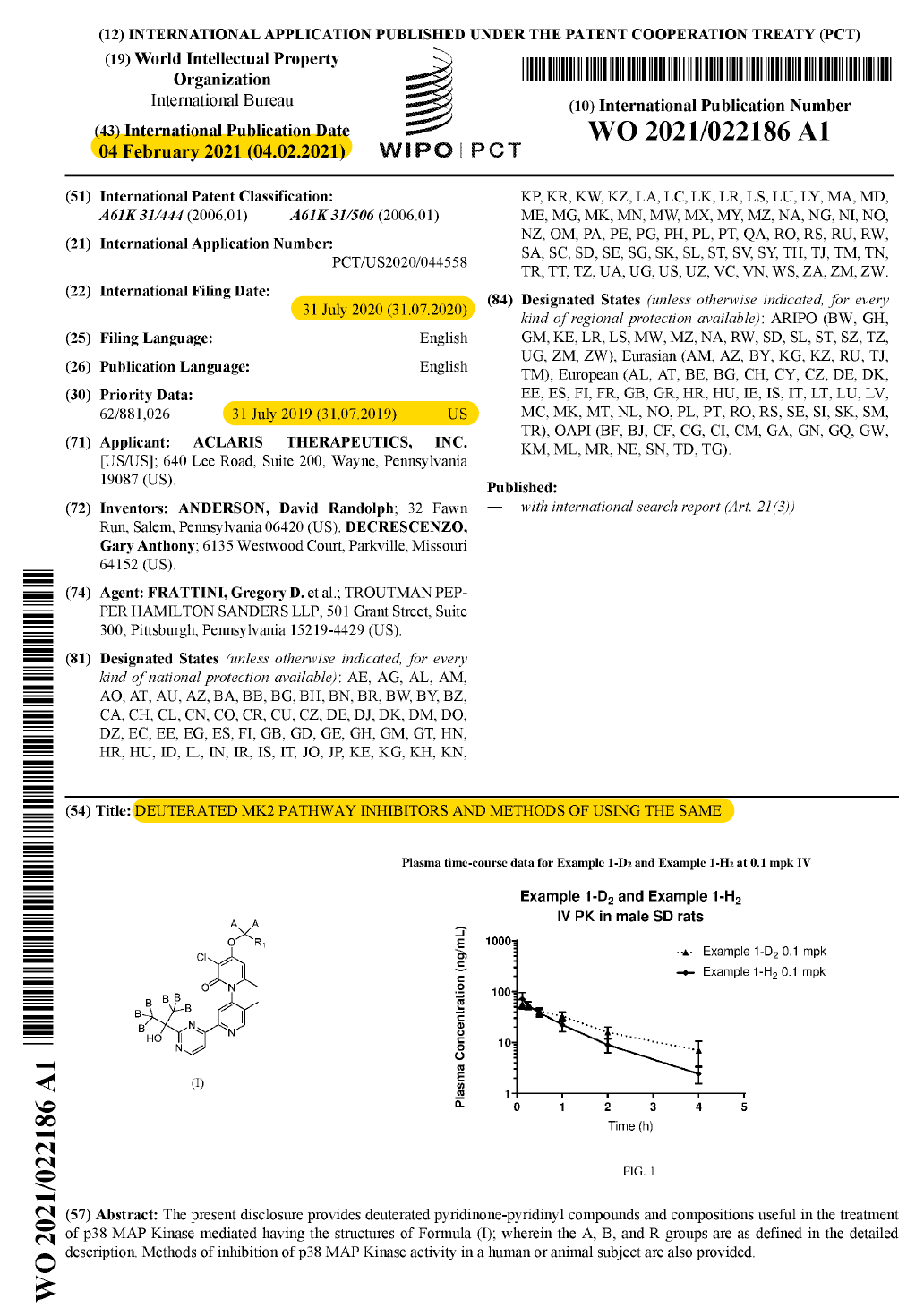
I came across this DEUTERATED MK2 inhibtors patent from Aclaris (WO2021022186A1) that was published in February 2021 (before the 3 patents on ATI-450 up there).
I skimmed it with skepticism. People sometimes use deuterium to try and get around patents and call it innovation. But sometimes, deuterium can alter PK for the better. As I looked through this patent, it appeared that Aclaris was trying to build the case for the latter.
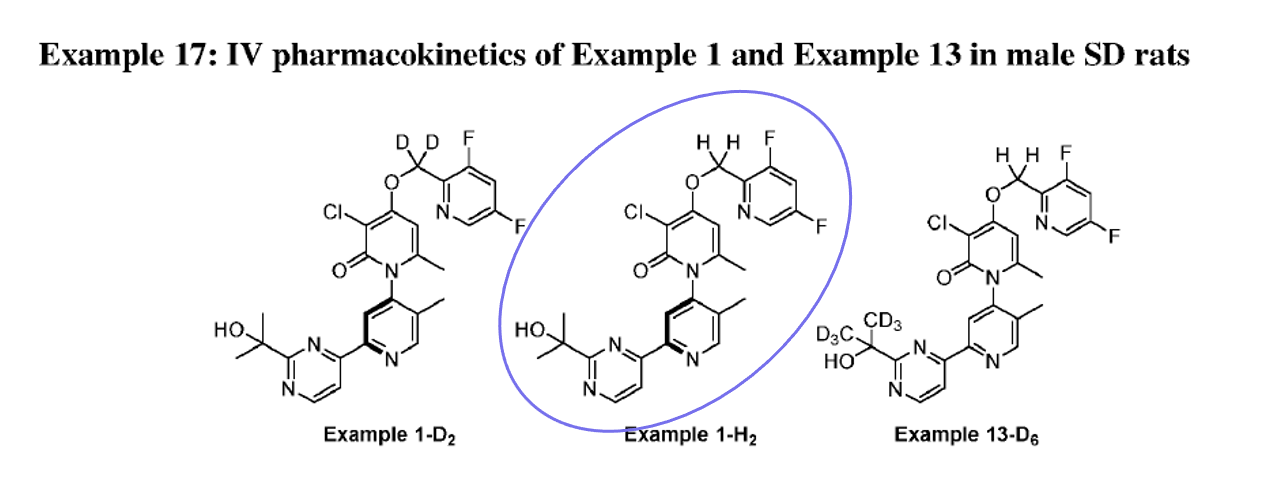
Basically, this patent describes the synthesis, some in vitro activity, and PK in rats for a series of 16 examples with deuterium substitutions at either the benzyl (D2) or dimethyl (D6) positions on structurally related pharmacophores.
As the cover figure suggests, plasma PK in rats compares PK of deuterated to the non-deuterated pharmacophore.
When I saw the PK data, I was excited! This is often the type of data that companies love to put in their slides. So I went to see what their company slides had to offer.
Nothing interesting in their company overview.
Nothing interesting in their Q1 2023 financial results.
But their Virtual R&D Day…what a gold mine!
The end is nigh!
Ctrl + F "2231” .
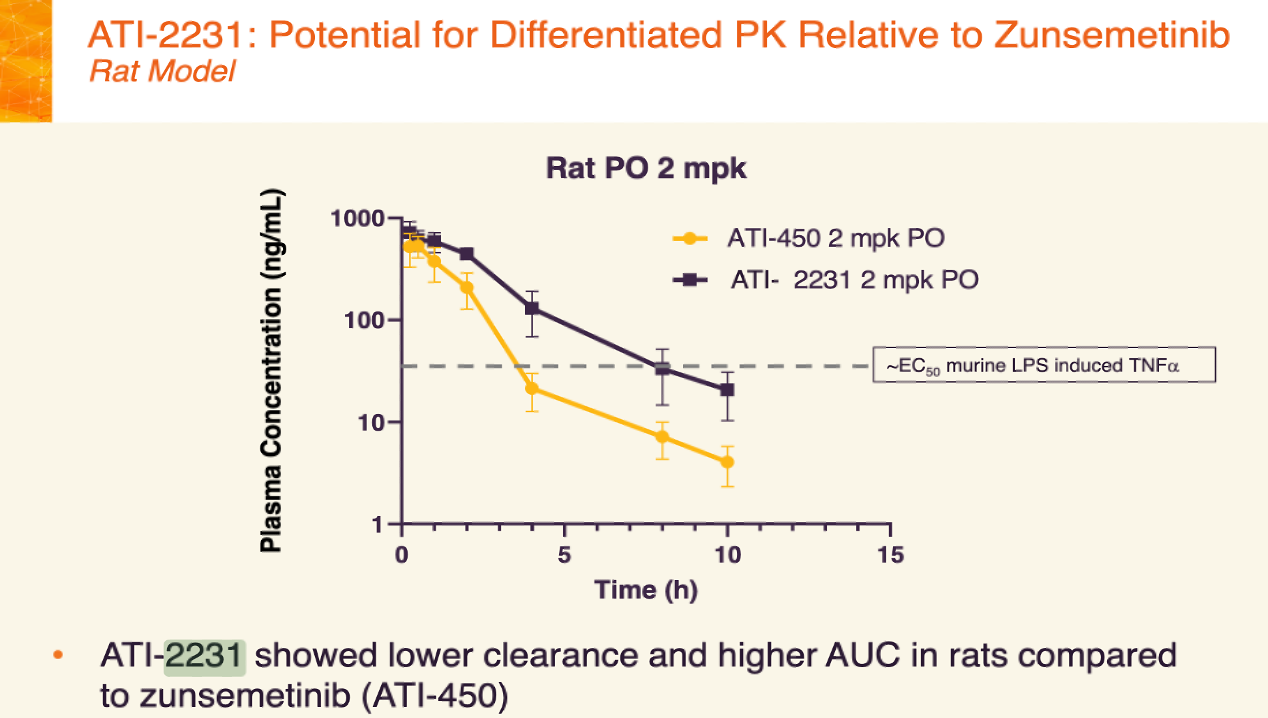
They got the rat PK, isolated enzyme data, in vitro selectivity data, cytokine inhibition, in vivo efficacy data in mice. Wow. What a wealth of info!
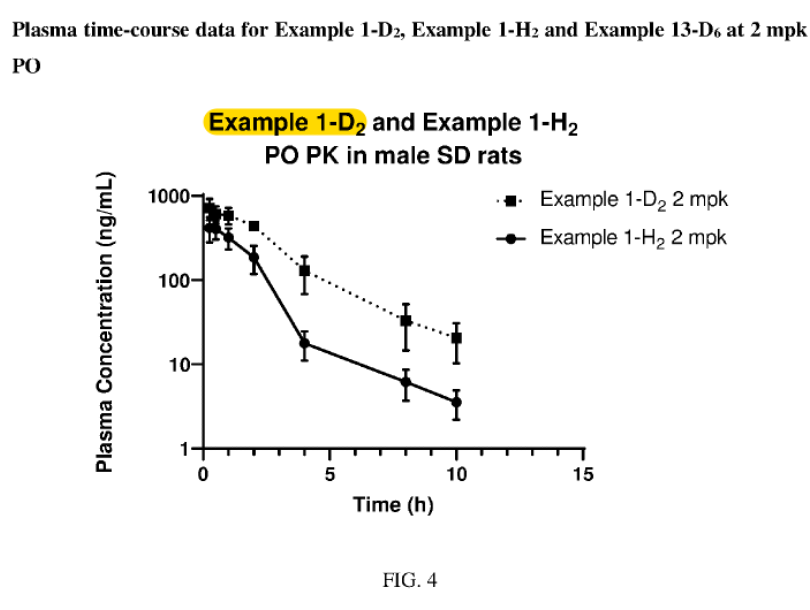
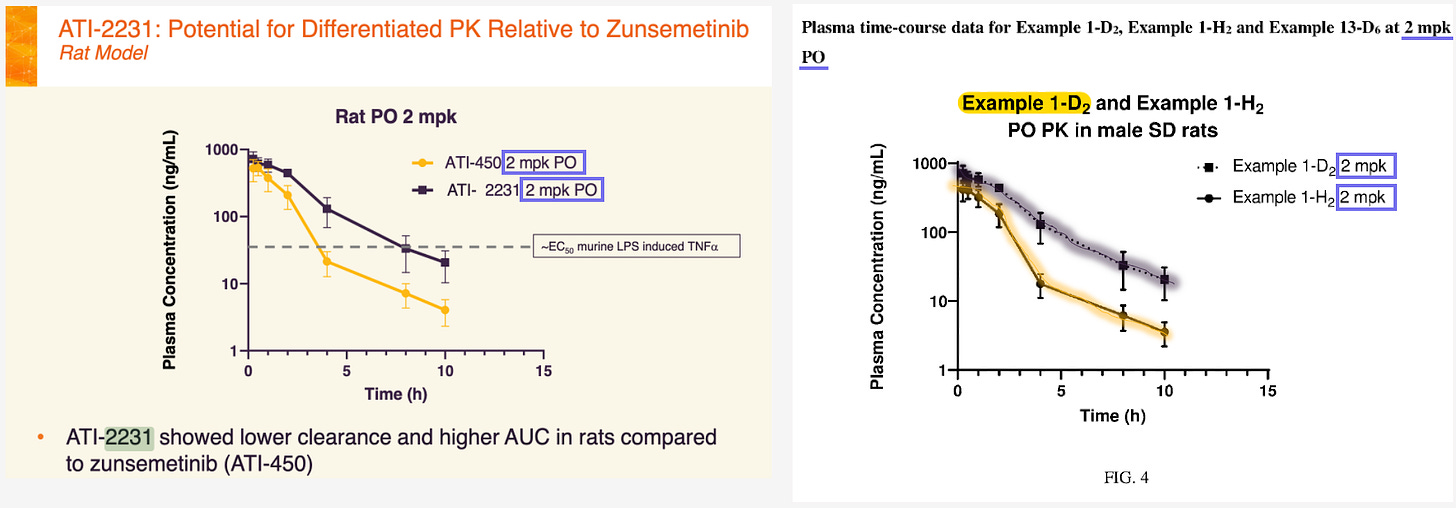
I knew the end was near when I saw the rat PK. ATI-450 vs. AT-2231 at 2 mpk.
Why? Because the deuterated MK2 inhibitors patent (‘186) was full of rat data. I had high hopes of finding a match!
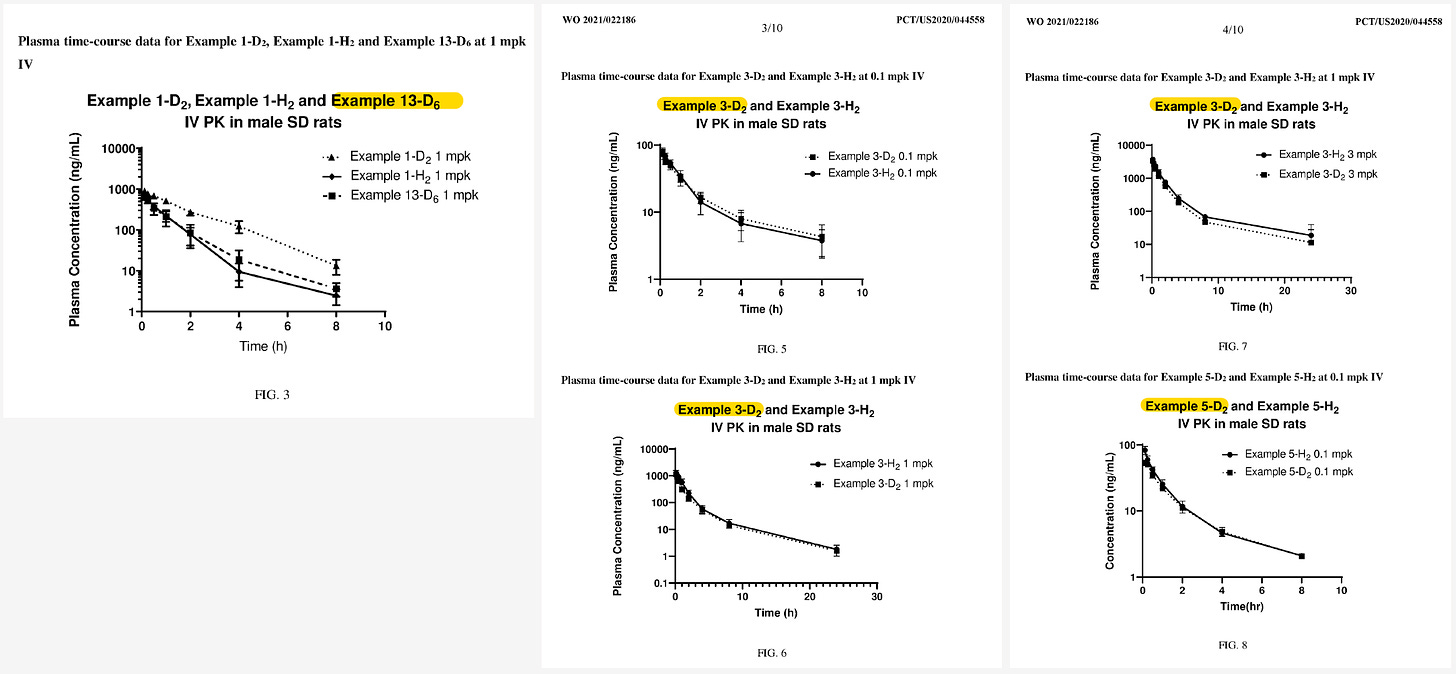
Most of the rat PK studies in the patent tested compounds at very low dose (0.1, 0.3, 1 mpk). So since the company slides showed data at 2 mpk, this would stand out in the patent graphs.
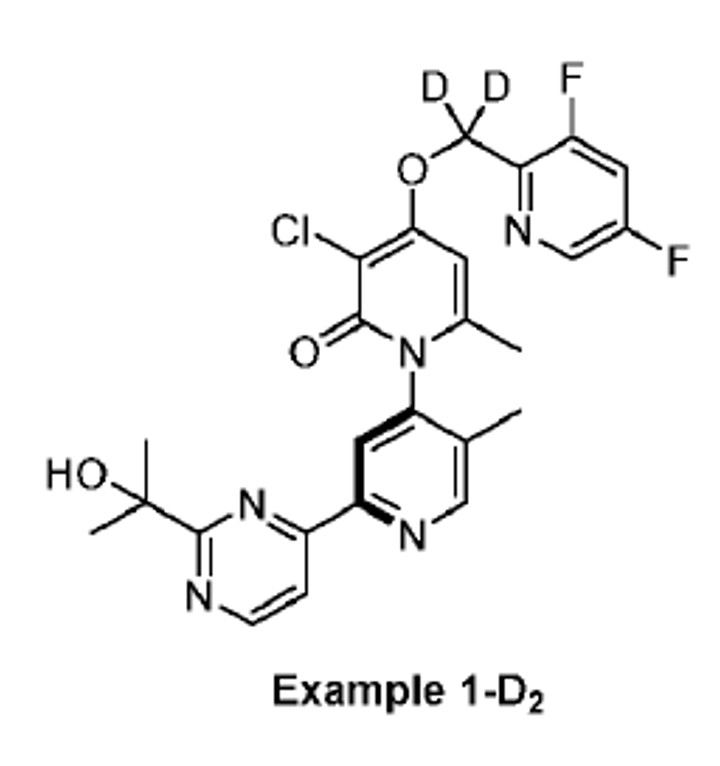
Note: in the deuterated patent, zunsemetinib (ATI-450) = “Example 1-H2”. On the leftmost graph, we can see that they compare a benzyl deuterated (D2) version of ATI-450 (“Example 1-D2) and a dimethyl deuterated (D6) version of ATI-450 (“Exmple 13-D6”).
Side note: interesting that, in these graphs, they compare deuterated pharmacophores to their non-deuterated counterparts rather than (or in addition to) comparing to ATI-450 which, presumably, is the compound they’re trying to beat as far as PK goes…
Anyway, I found the graph I was looking for—the one that compares the 2 compounds at 2 mpk!
Remember, Example 1-H2 = AT-450.
And if we put this graph right next to the comapny graph, we can see…!
They are the same! Right down to the error bar widths and axes.
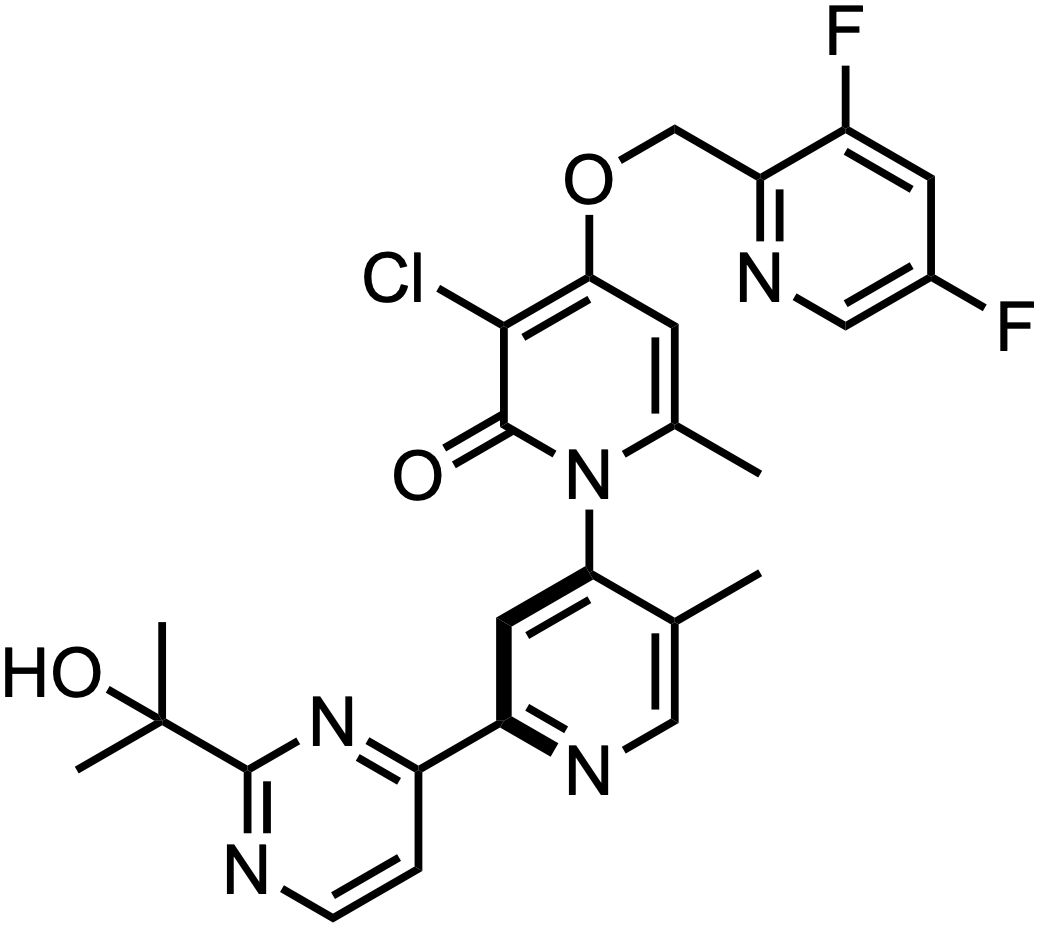
Therefore, ATI-2231 = Example 1-D2…
…the benzyl deuterated (D2) version of ATI-450!
Makes sense too, because the point of this was to improve PK.
Hope you enjoyed this episode of Molecular Sherlock! If you did, tell a friend about it :D Peace & see you in the next!
Have a compound that you want me look into? Suggest a compound here.