Confirmed correct on December 16, 2023 according to NCATS GSRS.
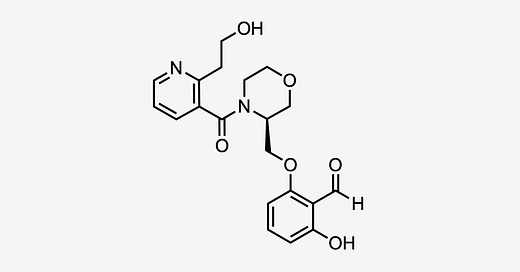
GBT-601 (aka GBT021601) is an oral hemoglobin S (HbS) polymerization inhibitor developed by Global Blood Therapeutics, a subsidiary of Pfizer (PFE 0.00%↑), that is currently in phase 2/3 trials (NCT05632354, NCT05431088) for the treatment of sickle cell disease (SCD). It is a 2nd generation HbS polymerization inhibitor that is supposed to be an improvement (lower dose, better PK) over the first-in-class compound, Oxbryta (voxelotor), which was FDA-approved for SCD in November 2019.