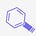
Confirmed correct on August 16, 2023 at ACS Fall National Meeting.
KIN-3248 is an irreversible, pan-fibroblast growth factor (FGFR) inhibitor by Kinnate Biopharma (KNTE 0.00%↑) that is currently in phase 1 to treat patients with advanced tumors harboring FGFR2/3 mutations (NCT05242822).