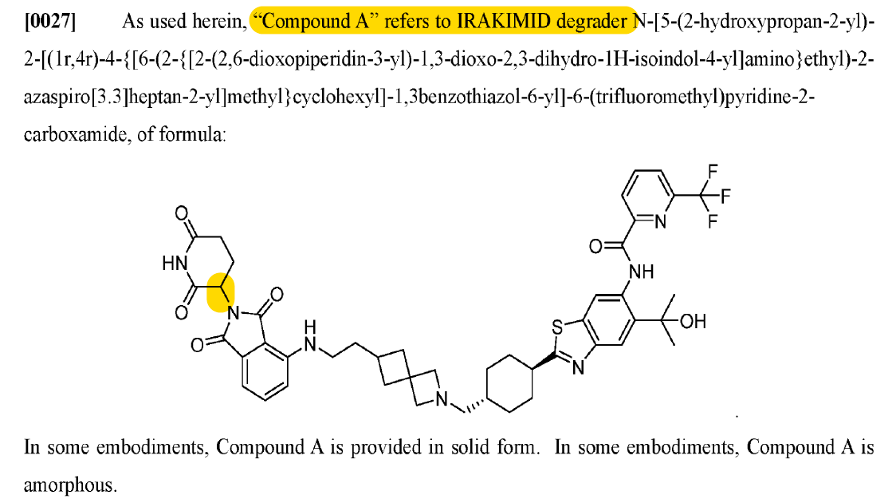
Confirmed correct on June 26, 2024 via Journal of Medicinal Chemistry.
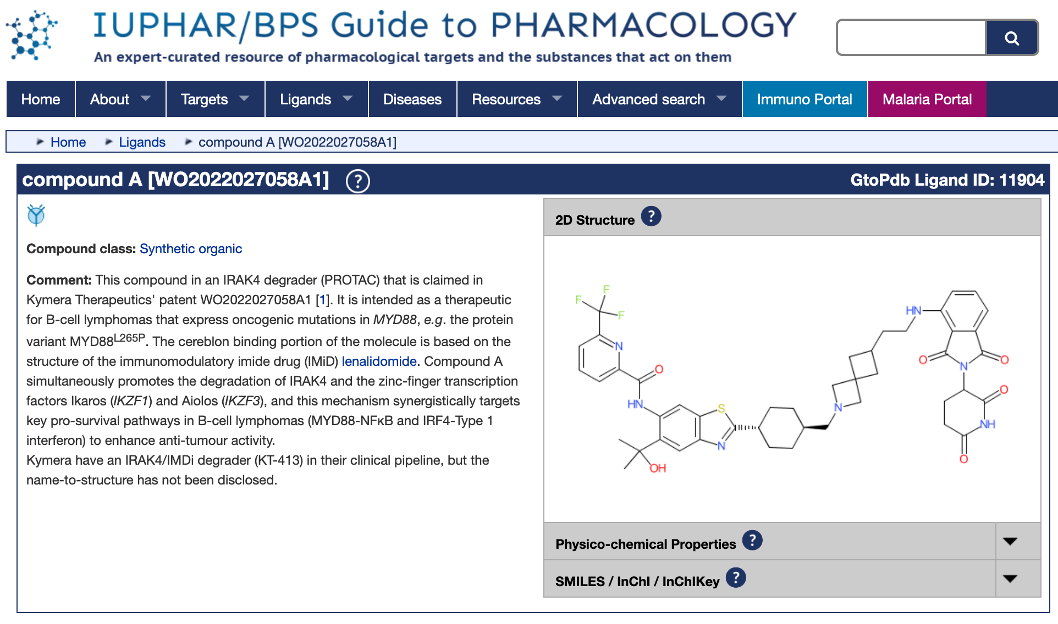
KT-413 is an intravenously administered heterobifunctional IRAKIMiD degrader developed by Kymera Therapeutics (KYMR 0.00%↑) that is currently being evaluated for the treatment of relapsed/refractory B-cell non-Hodgkin’s lymphoma (NHL) in ph1 (NCT05233033).
I didn’t originally set out looking for KT-413…In fact, I was digging around to find KT-474, the IRAK4 degrader that Kymera’s gonna present at the ACS fall meeting. But I managed to piece together KT-413 along the way, so might as well post!
KT-413 is a bifunctional degrader of IRAK4 and IMiD substrates, which are involved in pro-survival but redundant pathways in MYD88-mutant DLBCL.
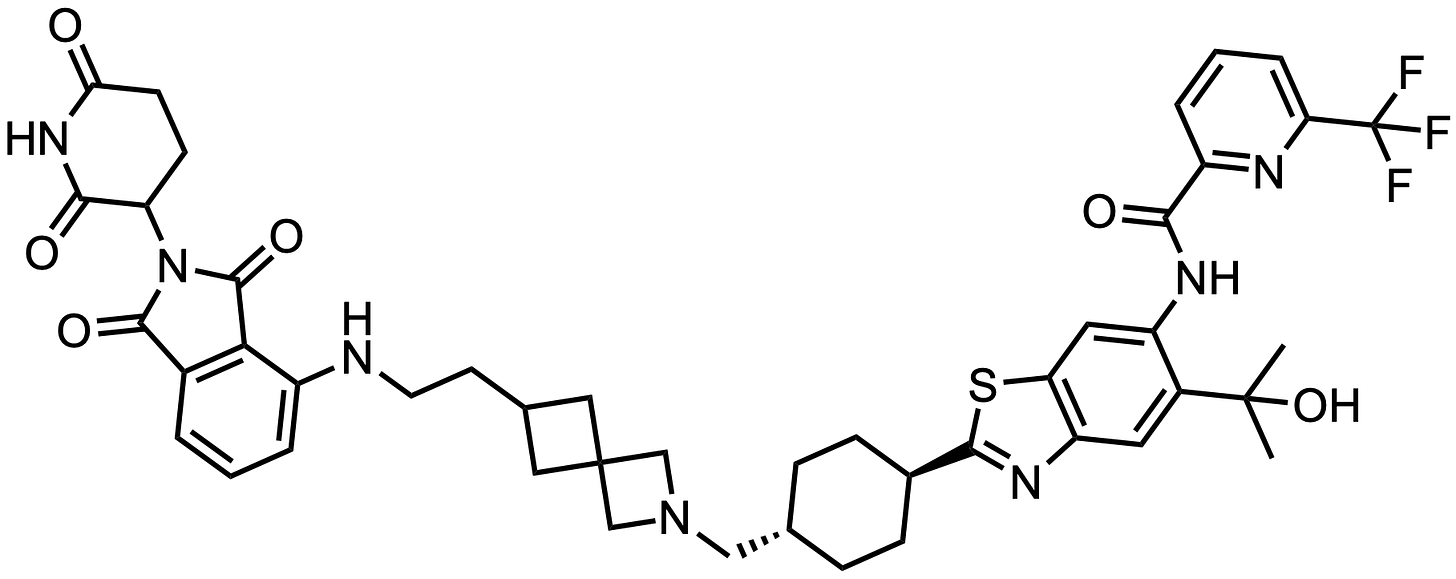
Heterobifunctional IRAKIMiD degrader
I’m more of a small molecules person, so degraders are a bit out of my wheelhouse. But I like seeing new things 👀. So we are here.
This all started how it usually starts: Espacenet. Searched “kymera therapeutics” and sorted by descending priority dates.
Because KT-474 (the compound that’s gonna be presented at ACS) is also an IRAK4 degrader, the very first hit caught my eye.
So I really do not know much about the specific goings on in the degrader space. But as I’ve mentioned previously, you really do not need to know the details of biology to figure these compounds out.
I knew that KT-474 was an IRAK4 degrader and, at the time, I hadn’t looked into Kymera’s pipeline. So I hadn’t known that they were also developing a bifunctional IRAKIMiD degrader that also targeted IRAK4 (aka, KT-413). So when I saw this patent, I had assumed it was going to contain the structure corresponding to KT-474.
I also hadn’t reviewed the proposed indication for KT-474 before jumping into this (lol). So the fact that the compound in this methods of use patent (‘439) was for B-cell NHL did not catch my attention…at first.
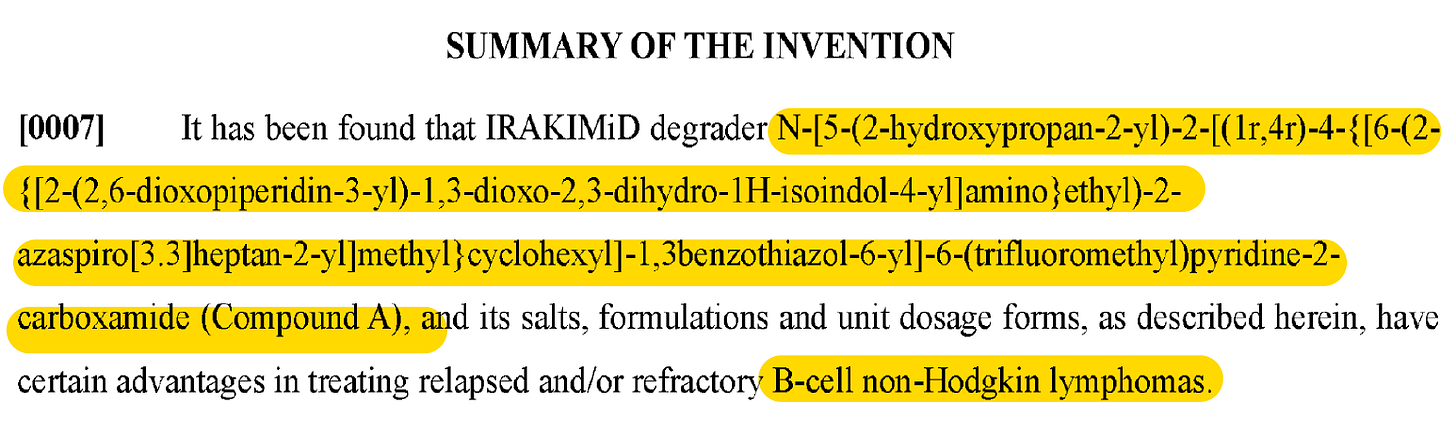
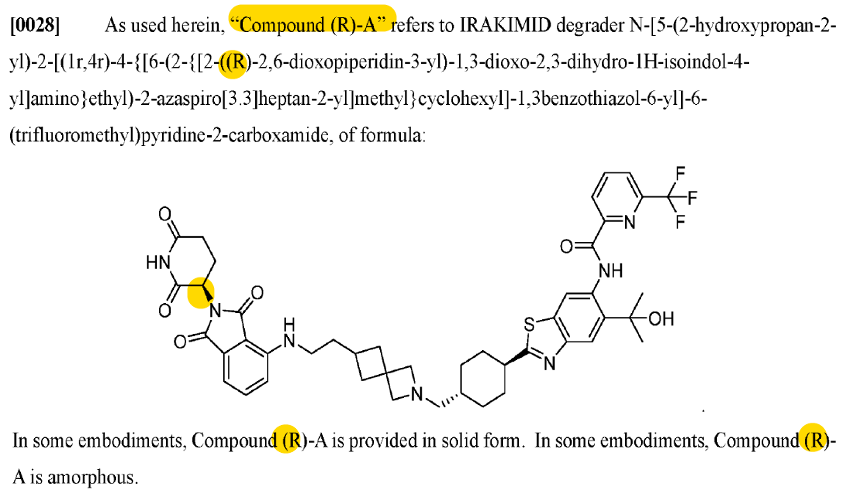
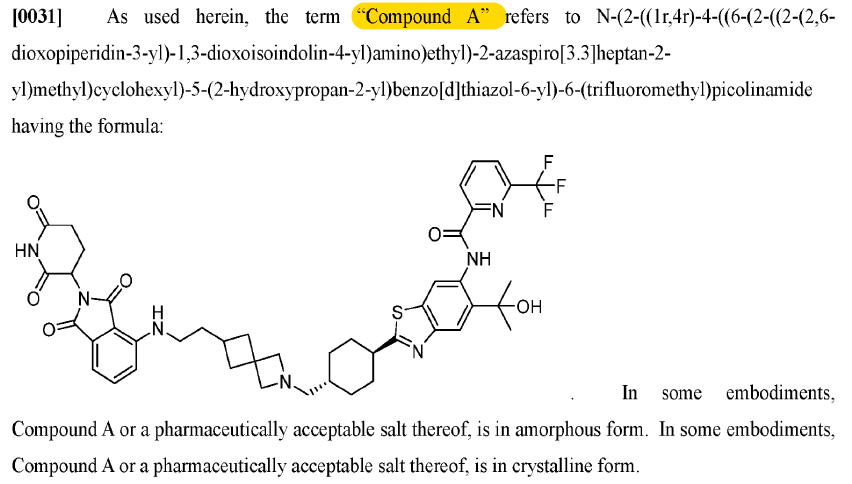
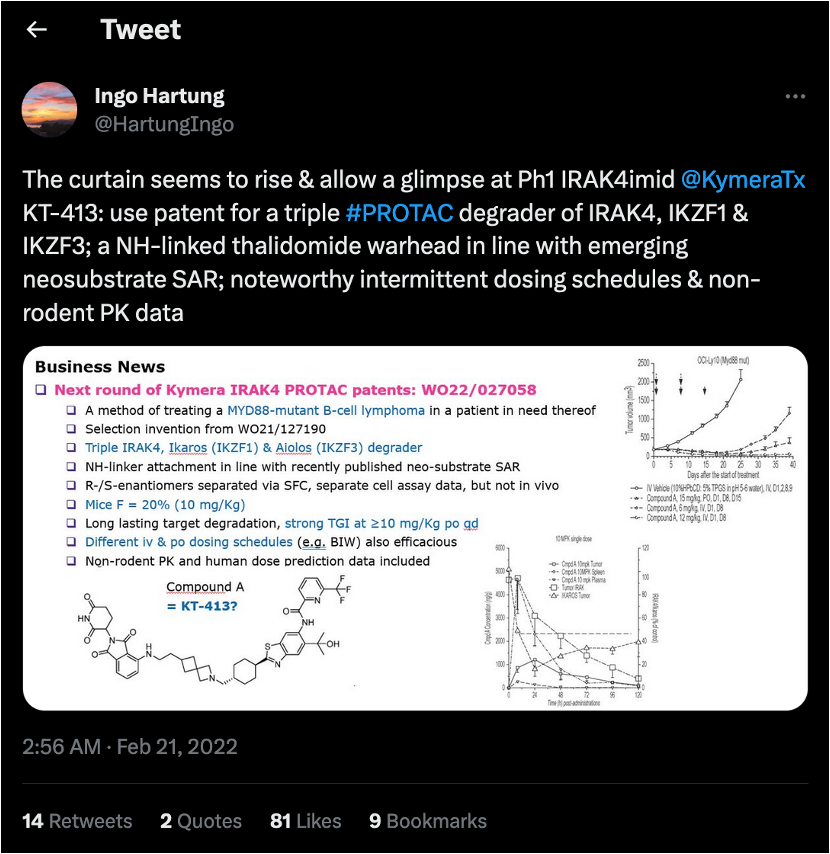
This patent (‘439) describes 1 compound (Compound A) and its R/S isomers. It’s a short patent that describes some results of a ph1 trial of Compound A (note: mix of isomers, not specifically Compound (R)-A or Compound (S)-A).
The first thing that tipped me off that this might not be KT-474 was the title of the clinical trial:
Upon seeing this example title, I searched the KT-474 trial on trials.gov—assuming that I would find the corresponding title for KT-474.
Wrong. Totally different indication and title.
Was Kymera developing another IRAK4 degrader??
I was perplexed, but kept reading through the ‘439 patent.
Interesting. So this “Compound A” in the ‘439 patent is an IV compound. But KT-474 is an oral one.
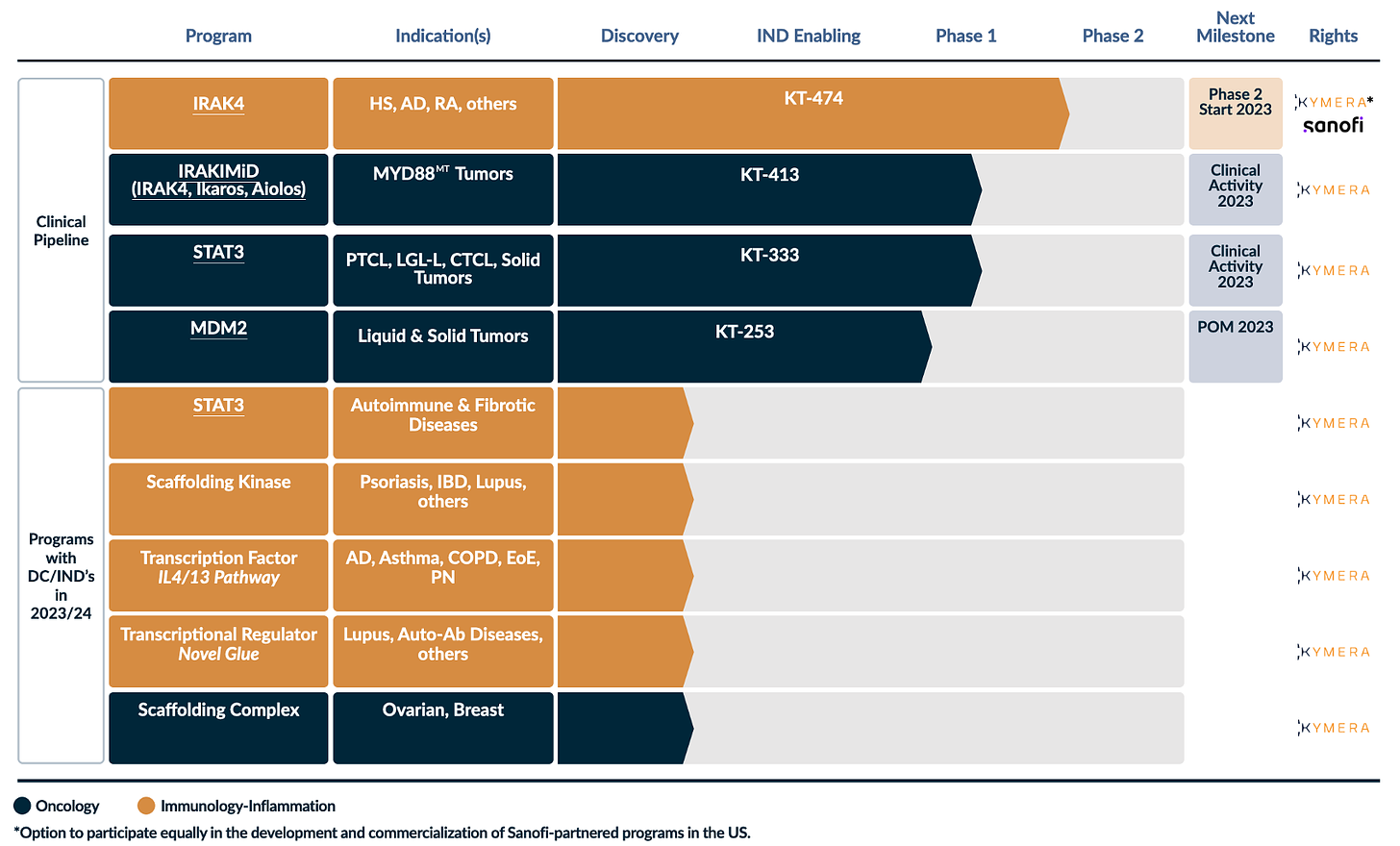
Not the same compound, it seemed. So then what could this mysterious Compound A be? I decided to go to the Kymera pipeline to see what my options were.
So this is when I realied that KT-474 is an IRAK4 degrader but KT-413 is an IRAKIMiD degrader. According to the pipeline, there are no other IRAK4 degraders in development.
So either Compound A in ‘439 is KT-413 or it is an abandoned compound that went through ph1 that Kymera recently patented (July 20, 2023 publish date).
Hm…what is more probable here?
Right before your very eyes
Ok so at this point I was running with the idea that this ‘439 Compound A may be KT-413. But I needed MORE PROOF.
I first searched “KT-413” on trials.gov. Aha!
Right indication this time! And just replace “Compound A” with “KT-413”.
And just to check that KT-413 is administered IV, as is done for Compound A in ‘439:
But you know me. I’m not gonna just call it a day after this because that is LAME AF. We need MORE!
So I went searching the ISR for some possible clues. This led me to 3 patents:
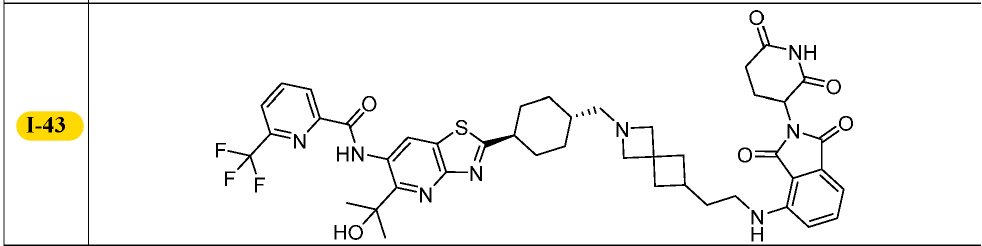
I checked the first listed patent (WO 2021127190) because they cited a specific example (exemplary compound I-43). This would (hopefully) make things a bit easier for me.
Indeed, I-43 was the same compound as Compound A in ‘439. Comforting. But the in vitro data in this patent was totally unhelpful because they did the (annoying) pharma thing where they rate the compound as “A” and say that its potency is < 0.5 µM when the company slides say the compound is actually in the nM range. So these ratings are basically useless for me.
Moving on…!
I had a feeling that the rest of the patents in the ISR were not gonna help me. So I decided to just scroll through the rest of the “kymera therapeutics” Espacenet hits to try my luck.
BTW, at this point I had already sifted through various company presentations, so I had some vague memory of the types of data available for me to match. Some in vitro data, a lot of in vivo data, and some combo efficacy data in mice.
So hit 26 caught my eye because I already knew that KT-413 was being developed for lymphoma. This looked like another methods of use patent, so I hoped that it would have some in vivo data because I knew the company slides had in vivo data on KT-413.
BOOMBAYAH
So this is where things all come together.
At the very end of this methods of use in lymphoma patent (‘058) are where all the good figures are. And there is a lot of in vivo data as monotherapy or in combination.
And as it turns out, Kymera presented on at the International Conference on Malignant Lymphoma in June 2021 on KT-413 single agent and combo efficacy in various mouse models.
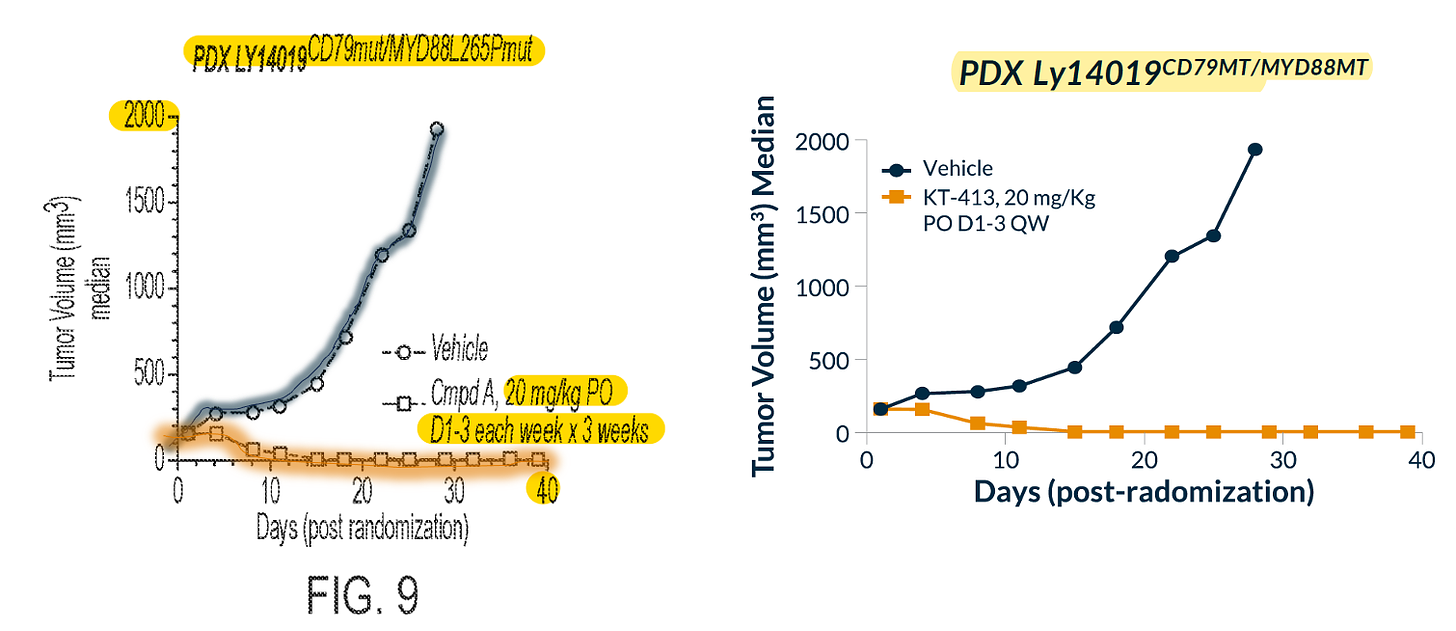
FIRST: mono-activity of Compound A (left, ‘058 patent) and KT-413 (right, company presentation) in LY14019 CD70MT/MYD88MT PDX. Same graph.
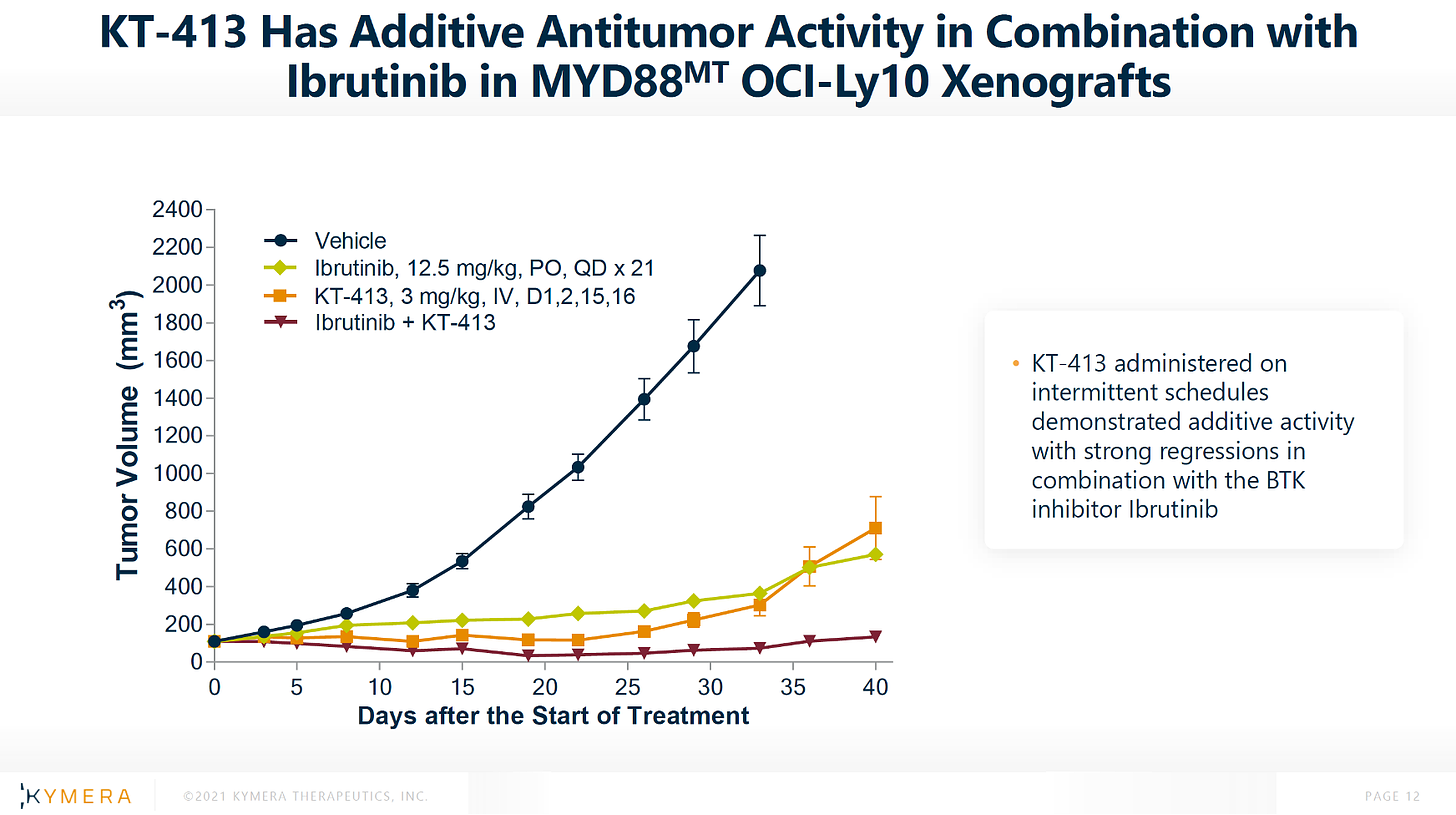
SECOND: KT-413 + ibrutinib in OCI-Ly10 xenografts.
Arm 1: ibrutinib dosed at 12.5 mpk PO QD for 21 days. Arm 2: Compound A dosed at 3 mpk IV on D1, 2, 15, 16. Arm 3: ibrutinib + Compound A. The same. Just switch “Compound A” for “KT-413”.
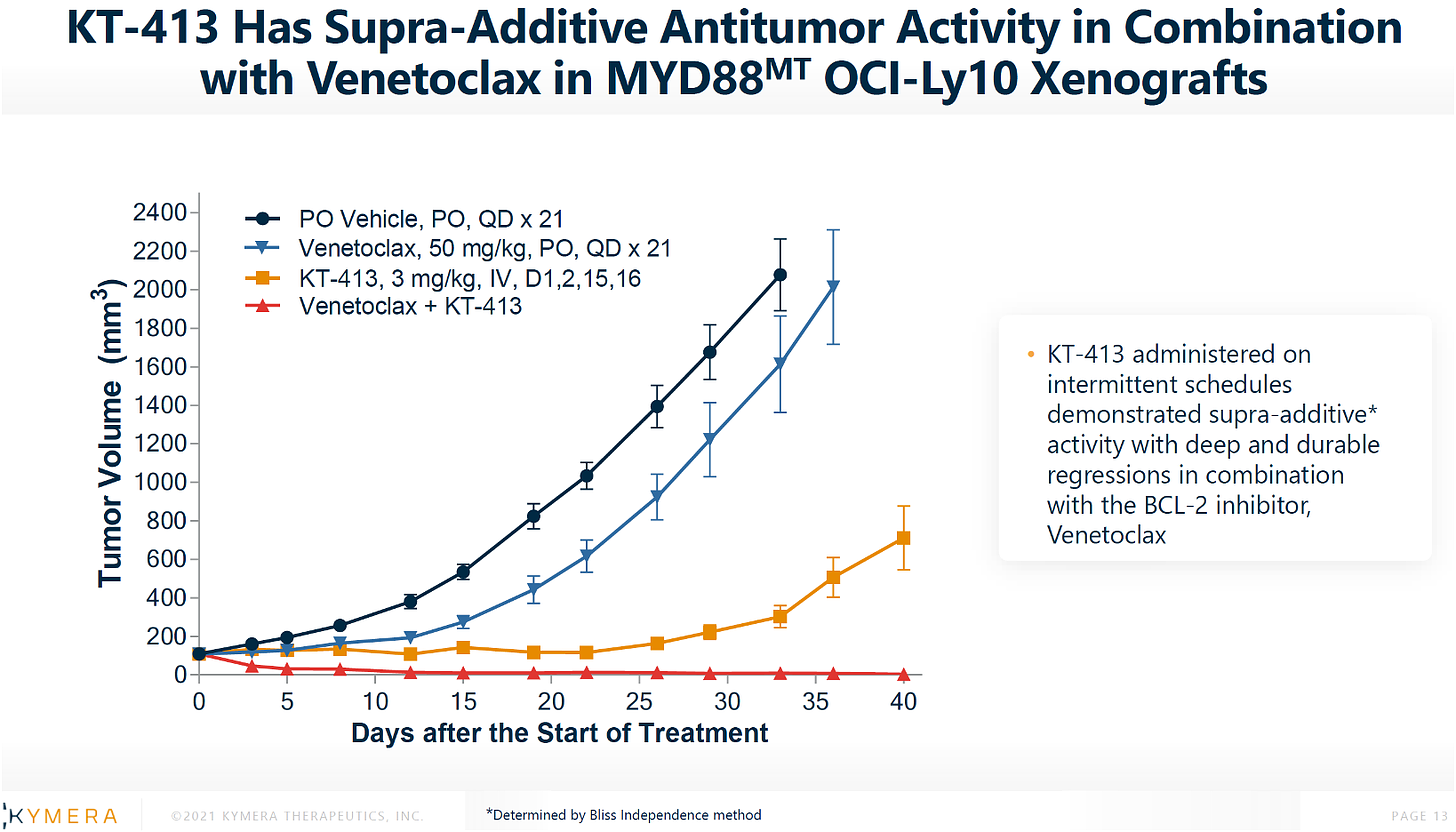
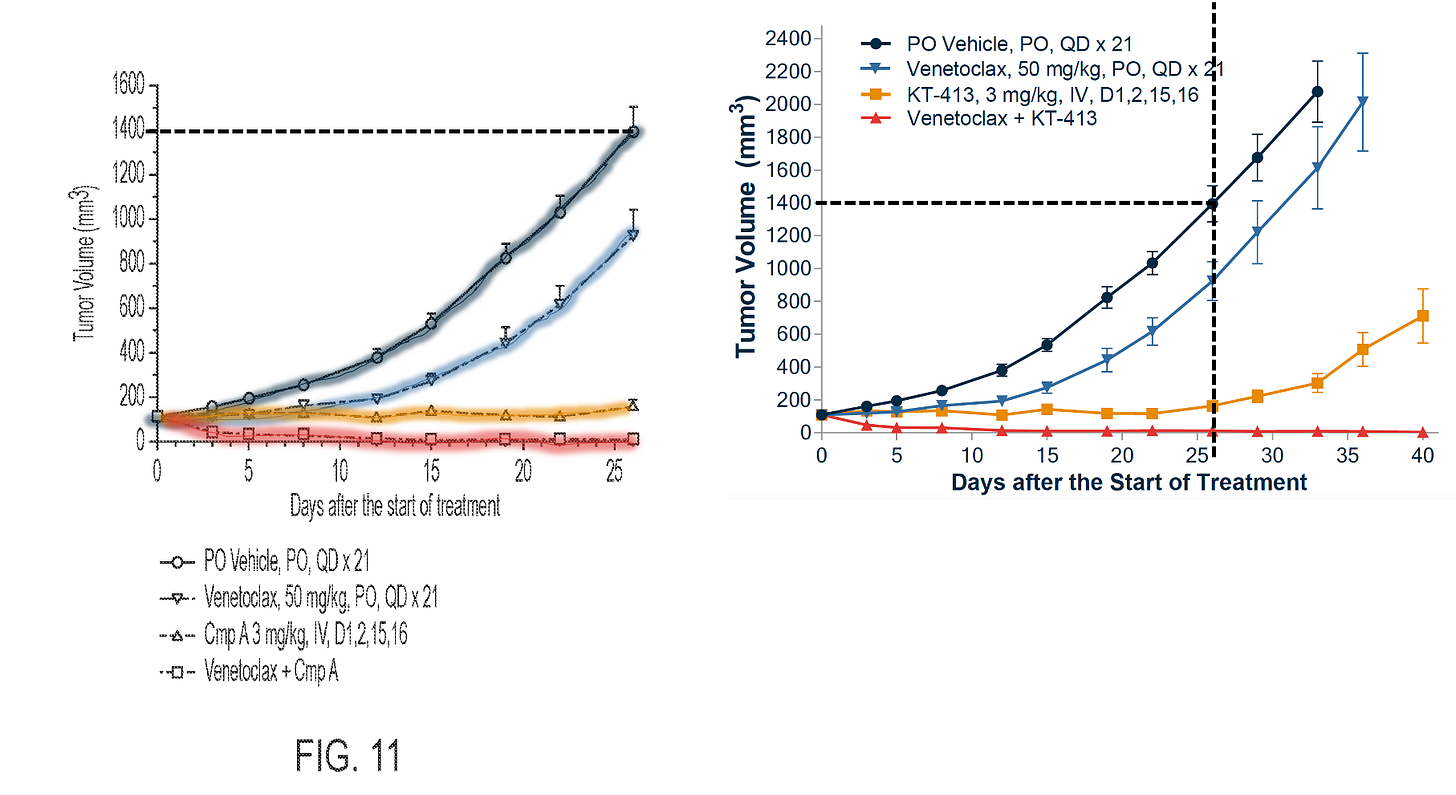
THIRD: KT-413 + venetoclax in MYD88MT OCI-Ly10 xenografts.
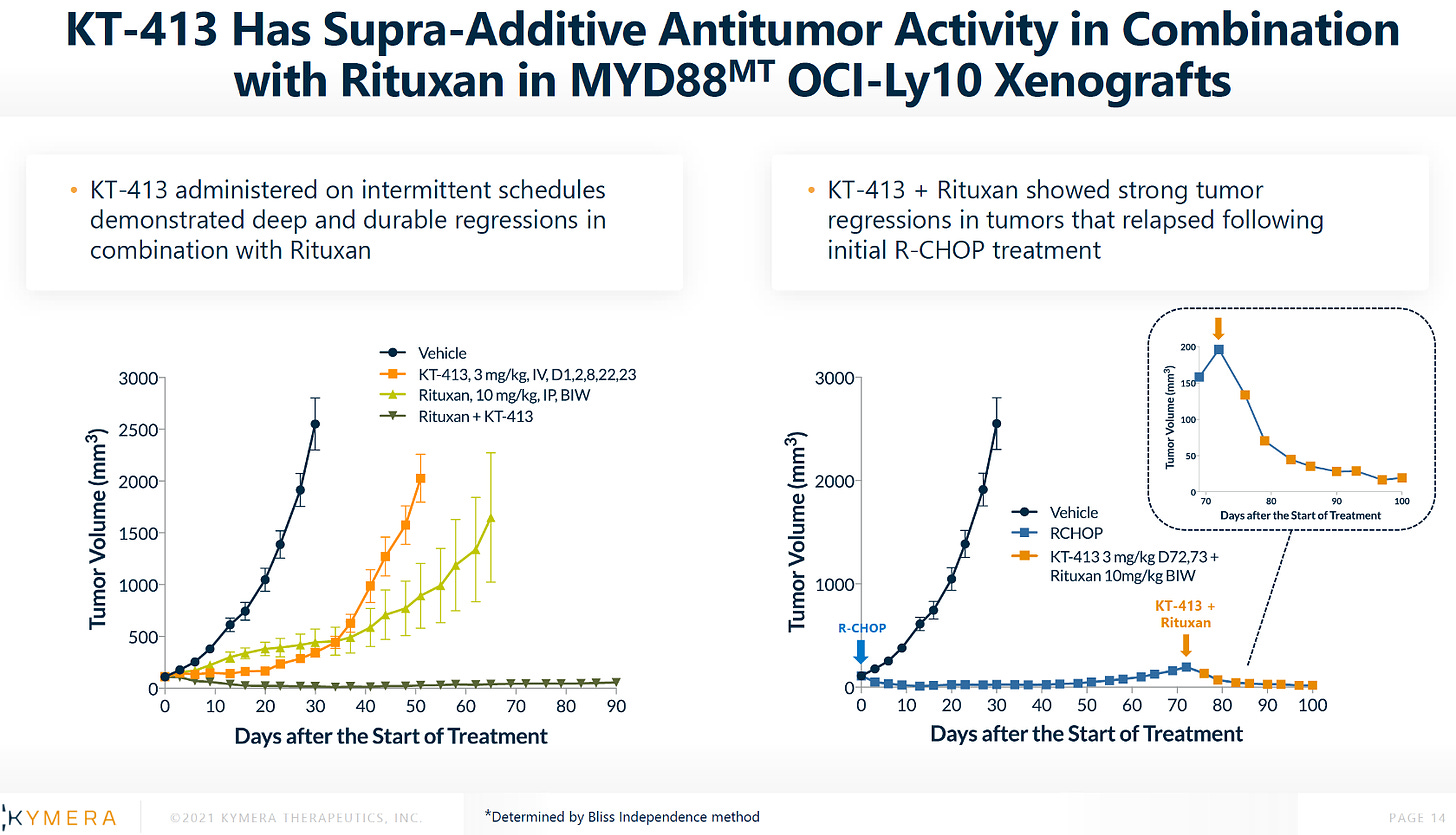
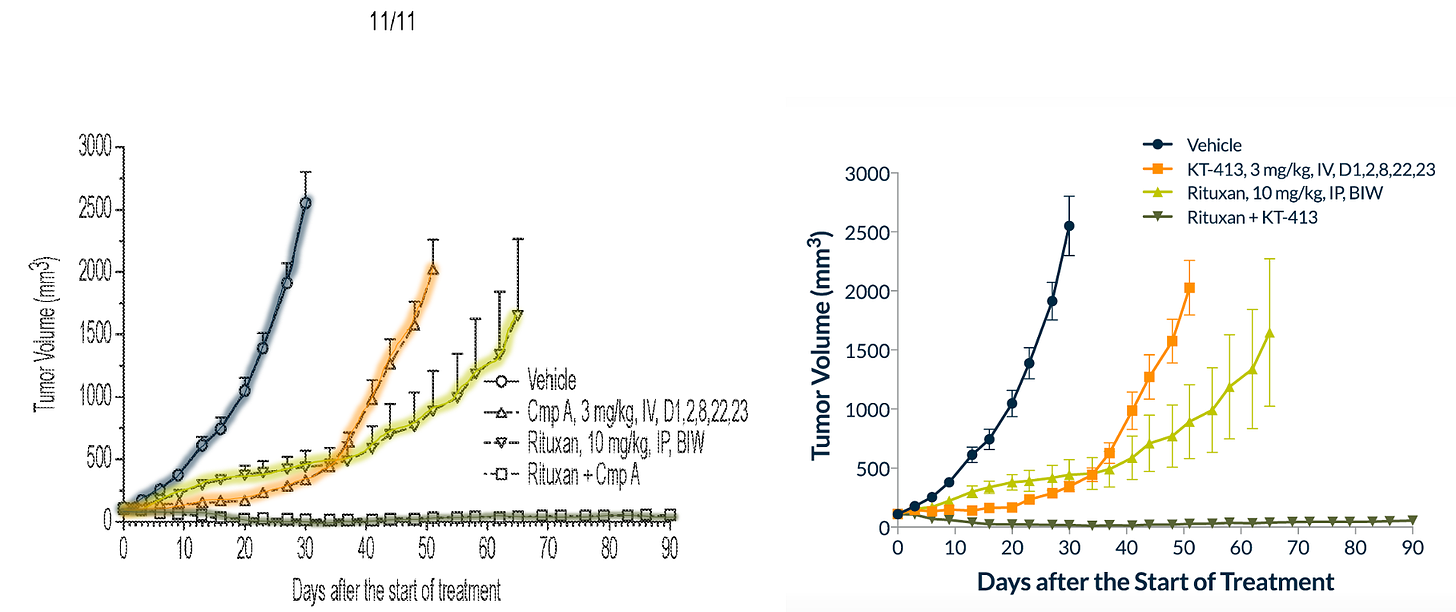
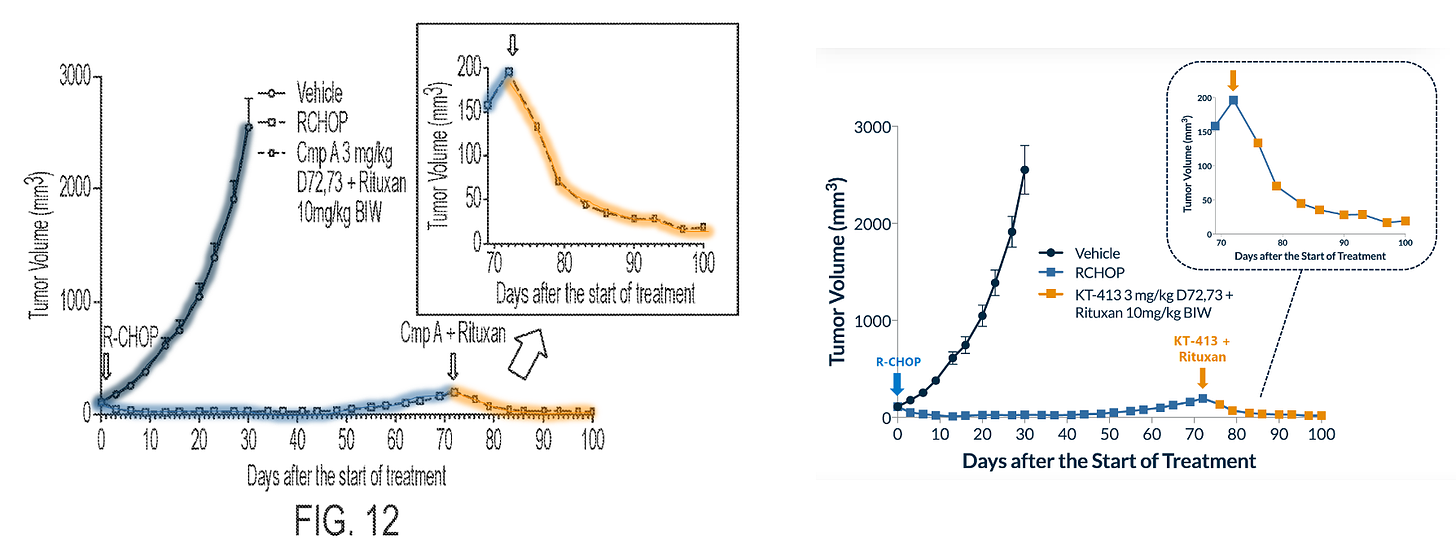
FOURTH: rituxan + KT-413 (left) and R-CHOP + KT-413 (right) in MYD88MT OCI-Ly10 xenografts.
Ok that was a lot. But you see my point?
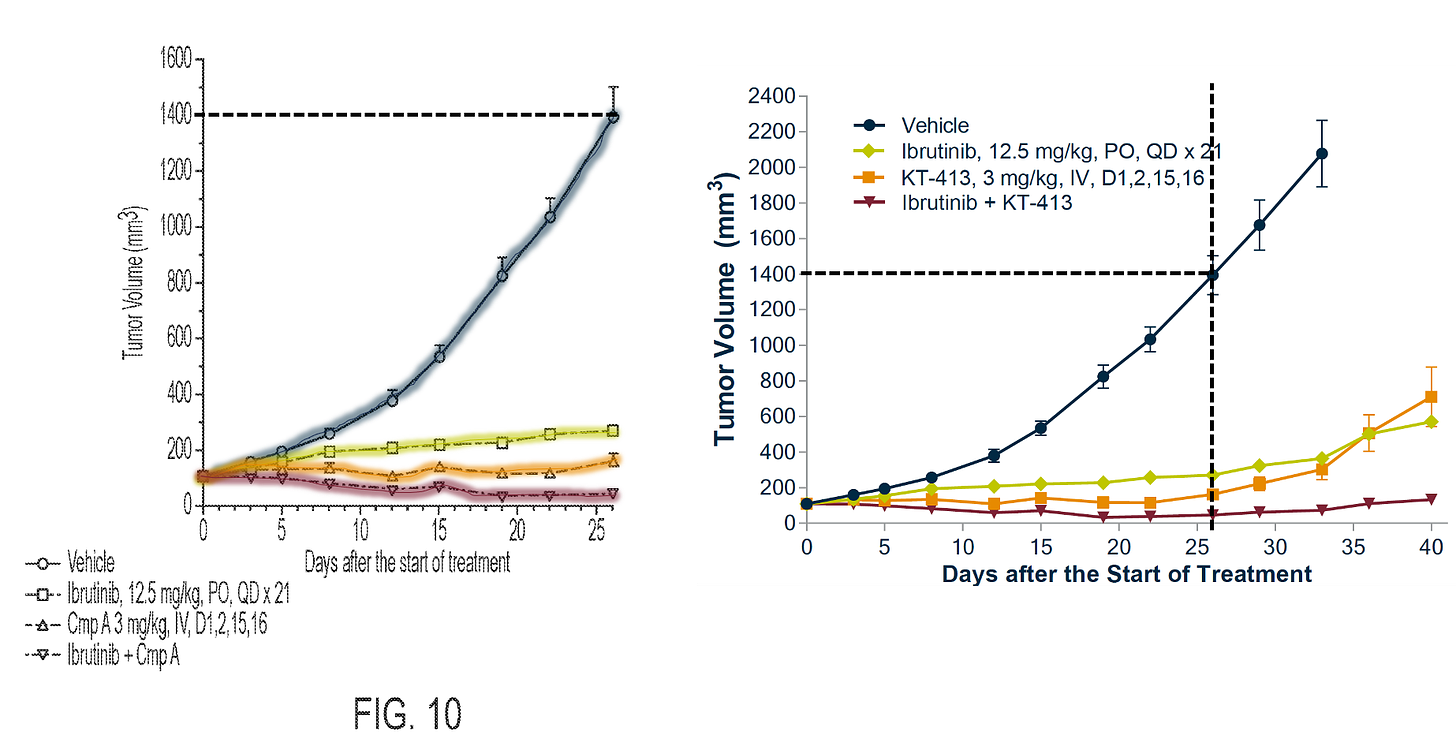
You might notice in some of the slides above that KT-413 was dosed PO in mice rather than IV, like what’s being done in the ph1 trial.
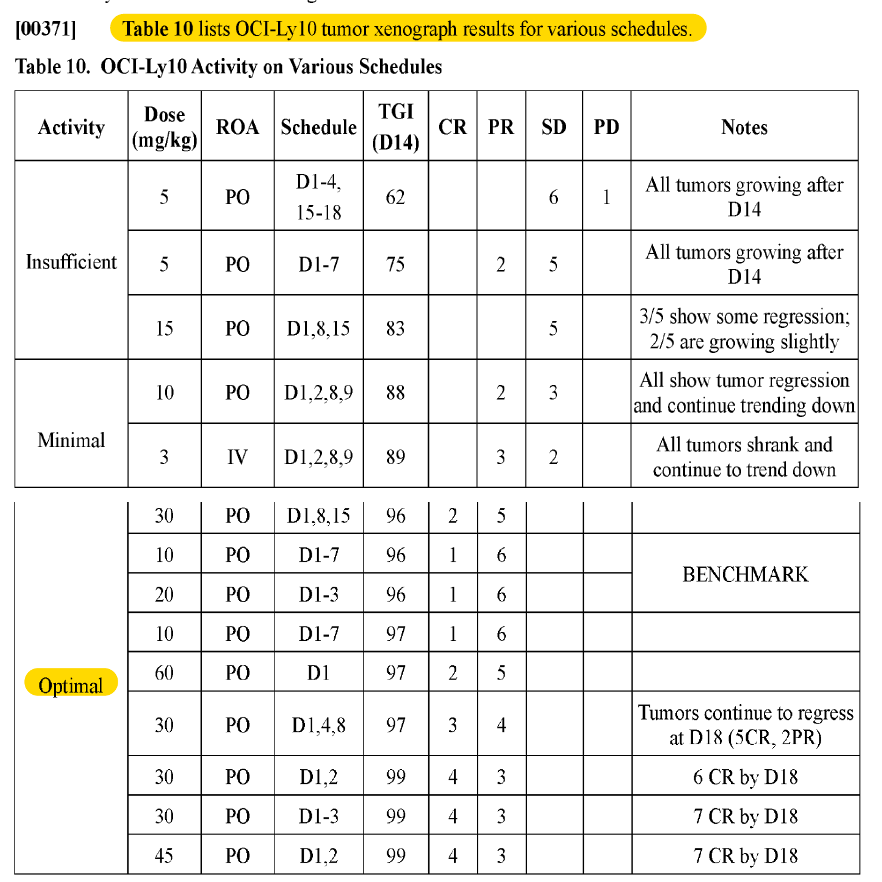
It turns out that they concluded that PO at the dose given was better than IV. Here’s a snippet of the Table 10 in the ‘058 patent, where they test various dosing regimens and routes of administration.
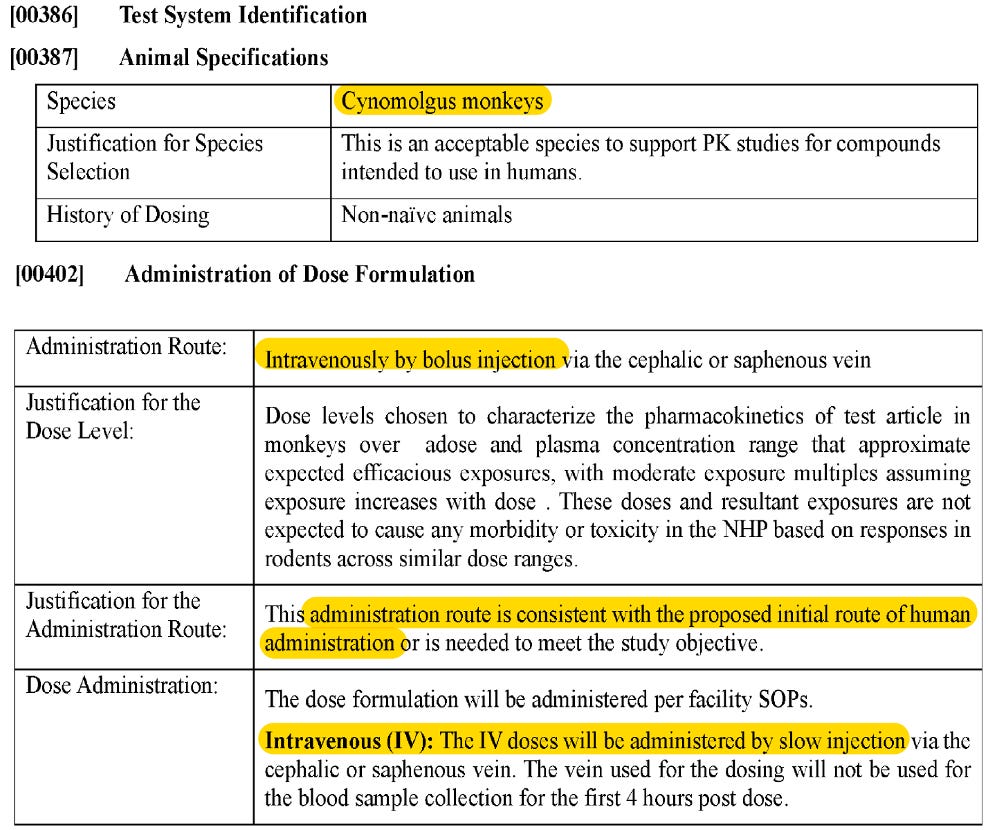
But later in the same ‘058 patent, when they assess PK in cynos, they test Compound A IV. Because this is the proposed route of administration for humans.
So there we have it! Compound A = KT-413!
The first degrader on Molecular Sherlock :)
P.S. I would be remiss if I didn’t mention that others have looked and have also identified this structure as KT-413. But here, I have provided very persuasive (imo) COMPRENSIVE PROOF tying it all together.
Have a compound that you want me look into? Suggest a compound here.