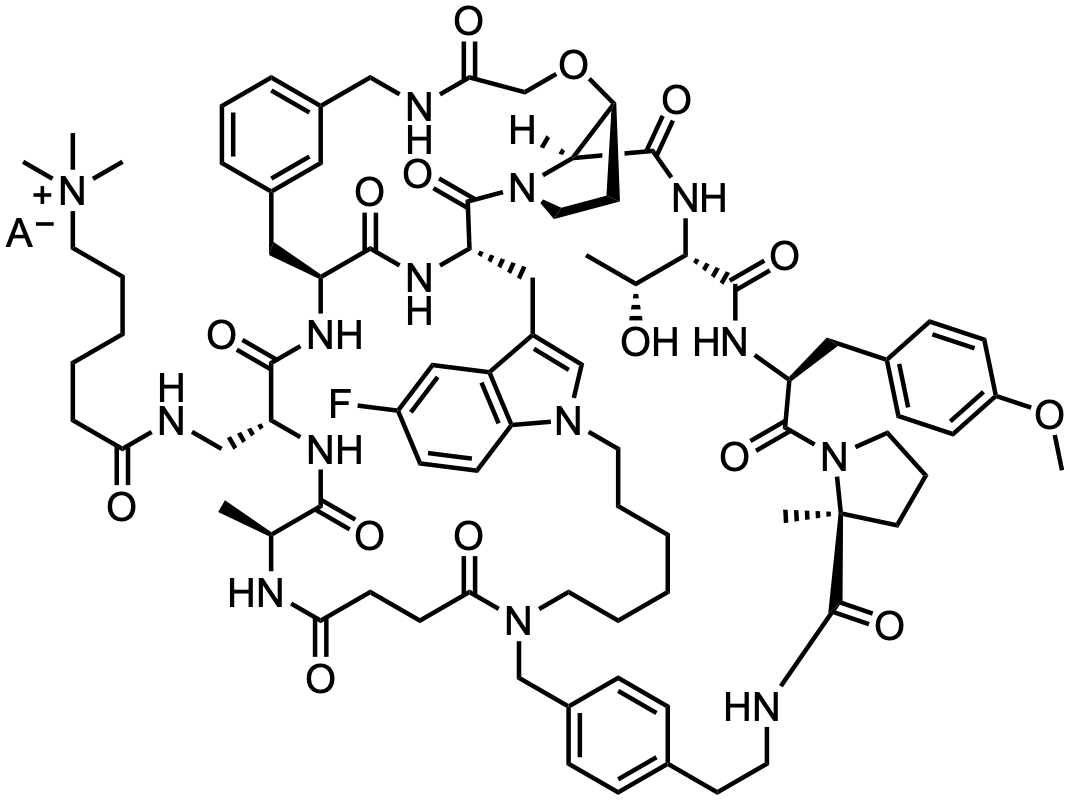
Confirmed correct on March 29, 2023 at ACS Spring 2023 First Time Disclosures.
MK-0616 is an oral proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor developed by Merck & Co. ( MRK 0.00%↑ ) for the treatment of hypercholesterolemia. It was recently evaluated in a phase 2 trial (NCT05261126) and a pivotal phase 3 trial is planned for the second half of …