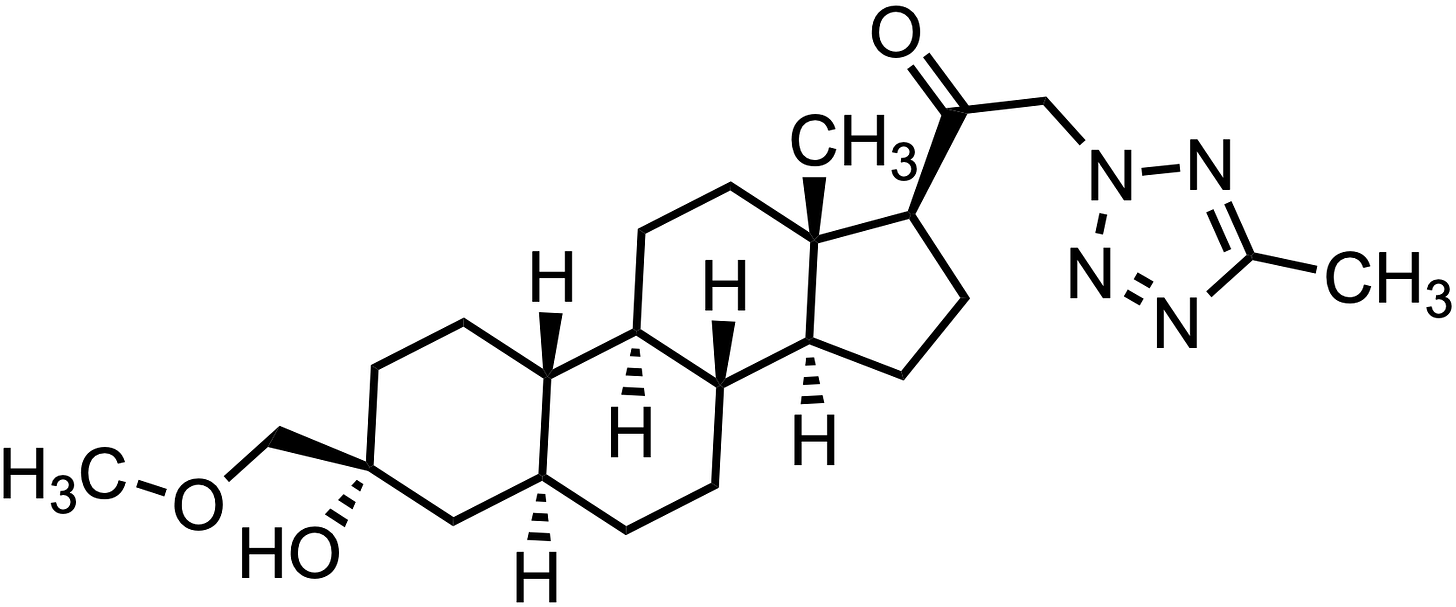
SAGE-324 (BIIB324) is an oral neurosteroid that acts as as a positive allosteric modulator (PAM) of the gamma aminobutyric acid-A (GABA-A) receptor. Currently it’s in phase 2/3 trials for the treatment of essential tremor (ET; NCT05366751, NCT05173012), the most common movement disorder. It is being co-developed by Sage Therapeutics (SAGE 0.00%↑) and Biogen (BIIB 0.00%↑) in the U.S. and by Biogen exclusively outside the U.S.
Substack is the home for great culture