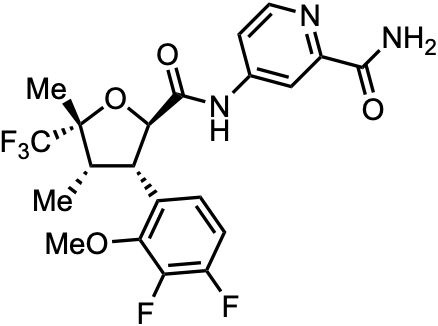
Note: Originally figured this one out on January 30, 2023. Structure was confirmed to be correct on February 1, 2023.
VX-548 is an oral NaV 1.8 inhibitor developed by Vertex Pharmaceuticals (VRTX 0.00%↑). It is currently in phase 3 trials as a non-opioid treatment for acute pain (NCT05661734, NCT05558410, NCT05553366). NaV 1.8 is a voltage-gated sodium ion channel involved in nociception.